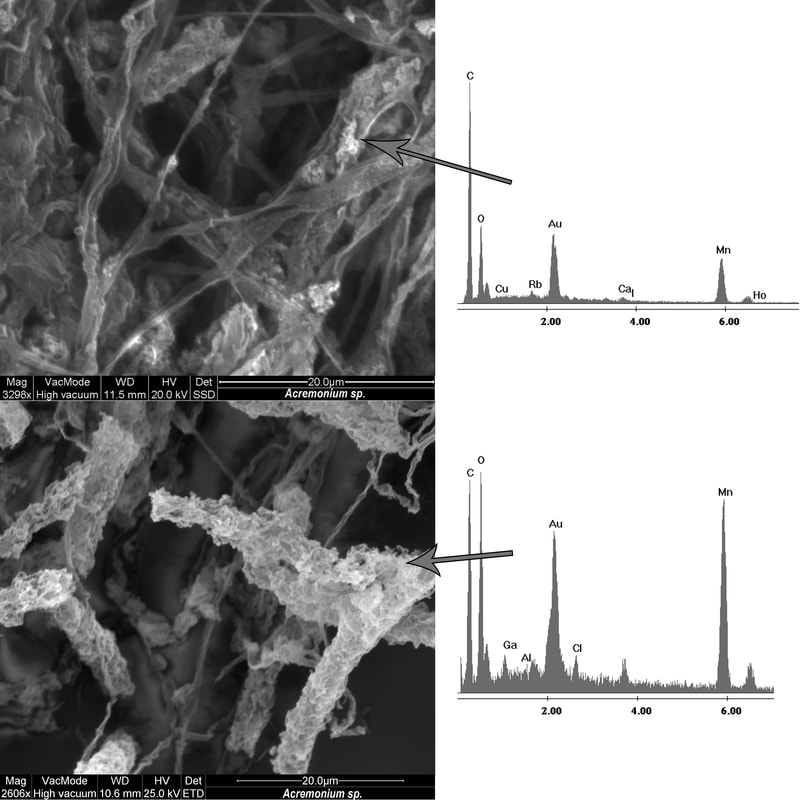
My MS research was focused on understanding the factors that constrained biotic Mn(II) oxidation in cave systems. Once oxidized, Mn oxides may be found coating the microbial cell or deposited away from the cell. These oxides can sequester other heavy metals, degrade carbon, or potentially protect microbes from antibiotics or reactive oxygen species. Many microbes including both bacteria and fungi have been found to oxidize Mn and appears to be dependent on the availability and type of carbon. This enzymatic process can be catalyzed by multicopper oxidases, superoxide dismutases, or Mn peroxidases.
Cave systems provide a unique opportunity to study microbe-mineral interactions in the environment because the systems are relatively controlled. Temperatures do not fluctuate over time, gases remain relatively constant, and large disturbance events are rare. Also, Mn oxidation naturally occurs in cave systems and is easy to test for in the field.
Cave systems provide a unique opportunity to study microbe-mineral interactions in the environment because the systems are relatively controlled. Temperatures do not fluctuate over time, gases remain relatively constant, and large disturbance events are rare. Also, Mn oxidation naturally occurs in cave systems and is easy to test for in the field.
Specific aims of this research project included:
1. Assess human impact on biotic Mn(II) oxidation in situ
This particular objective had two parts, A) determine how Mn(II)-oxidizing communities differed between caves with heavy human impact versus two caves with lower human impact, B) evaluate if carbon additions would stimulate Mn(II) oxidation, and C) assess how the Mn(II)-oxidizing communities change in response to the stimulation of oxidation via carbon additions.
There are two main ways that we hypothesized that humans can impact Mn(II) oxidation in caves and include direct impacts that occur during spelunking such as leaving behind litter, clothing fibers, and wood (brought in for fires) and indirect impacts such as polluting water sources that flow-through or flow above the cave.
One of the caves that we investigated had a lot of foot traffic, litter, and was connected to groundwater that had become polluted several years before my experiments started. The Mn oxides in this cave were extensive and occurred on the walls and floor.
The other two caves were more 'pristine', with only a few people visiting the cave each year, and no connections to groundwater. In these two caves, Mn oxides were more sparse.
Assessment of the carbon content of the water sources for each cave indicated that there was a seasonal increase in DOC but the magnitude of increase was most significant in the cave with historical documentation of anthropogenic disturbances.
2. Determine if host-rock geochemistry is correlated with Mn(II)-oxidizing communities
For this objective, we were interested to evaluate if Mn(II) oxidizing communities, including both bacterial/archaeal and fungal, were related to host-rock geochemistry. The caves that were studied were found in the southeastern US along the Appalachian Mountains. While they were all within relatively close proximity to one another, they had different host-rock geochemistries.
3. Investigate the geochemical constraints on biotic Mn(II) oxidation in caves
For this objective, our analyses focused on the impact of Sr and Cu. At the ONE site where Mn(II) oxidation was not stimulated, Sr was several magnitudes higher than any other site. So, we tested the effect of high Sr concentrations on the ability of isolates to oxidize Mn(II).
Previous studies have indicated the potential importance of Cu in constraining biotic Mn(II) oxidation in situ and have hypothesized that these constraints were largely due to the mechanism of oxidation. Superoxide dismutases have a higher affinity for Cu compared to Mn, therefore Cu would be complexed rather than Mn. In contrast, Cu can stimulate multicopper oxidase Mn(II) oxidation. Therefore, carbon additions containing Cu would help to elucidate the dominant enzymatic pathway used by bacteria to oxidize Mn.
This particular objective had two parts, A) determine how Mn(II)-oxidizing communities differed between caves with heavy human impact versus two caves with lower human impact, B) evaluate if carbon additions would stimulate Mn(II) oxidation, and C) assess how the Mn(II)-oxidizing communities change in response to the stimulation of oxidation via carbon additions.
There are two main ways that we hypothesized that humans can impact Mn(II) oxidation in caves and include direct impacts that occur during spelunking such as leaving behind litter, clothing fibers, and wood (brought in for fires) and indirect impacts such as polluting water sources that flow-through or flow above the cave.
One of the caves that we investigated had a lot of foot traffic, litter, and was connected to groundwater that had become polluted several years before my experiments started. The Mn oxides in this cave were extensive and occurred on the walls and floor.
The other two caves were more 'pristine', with only a few people visiting the cave each year, and no connections to groundwater. In these two caves, Mn oxides were more sparse.
Assessment of the carbon content of the water sources for each cave indicated that there was a seasonal increase in DOC but the magnitude of increase was most significant in the cave with historical documentation of anthropogenic disturbances.
2. Determine if host-rock geochemistry is correlated with Mn(II)-oxidizing communities
For this objective, we were interested to evaluate if Mn(II) oxidizing communities, including both bacterial/archaeal and fungal, were related to host-rock geochemistry. The caves that were studied were found in the southeastern US along the Appalachian Mountains. While they were all within relatively close proximity to one another, they had different host-rock geochemistries.
3. Investigate the geochemical constraints on biotic Mn(II) oxidation in caves
For this objective, our analyses focused on the impact of Sr and Cu. At the ONE site where Mn(II) oxidation was not stimulated, Sr was several magnitudes higher than any other site. So, we tested the effect of high Sr concentrations on the ability of isolates to oxidize Mn(II).
Previous studies have indicated the potential importance of Cu in constraining biotic Mn(II) oxidation in situ and have hypothesized that these constraints were largely due to the mechanism of oxidation. Superoxide dismutases have a higher affinity for Cu compared to Mn, therefore Cu would be complexed rather than Mn. In contrast, Cu can stimulate multicopper oxidase Mn(II) oxidation. Therefore, carbon additions containing Cu would help to elucidate the dominant enzymatic pathway used by bacteria to oxidize Mn.
|
Above is an SEM image of two isolates that were oxidizing Mn(II) and coating the fungal hyphae with the oxides.
|